Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to study the properties and interactions of atomic nuclei in molecules. It provides detailed information about the local magnetic environments of specific atomic nuclei, which can be used to determine molecular structures, dynamics, and other properties.
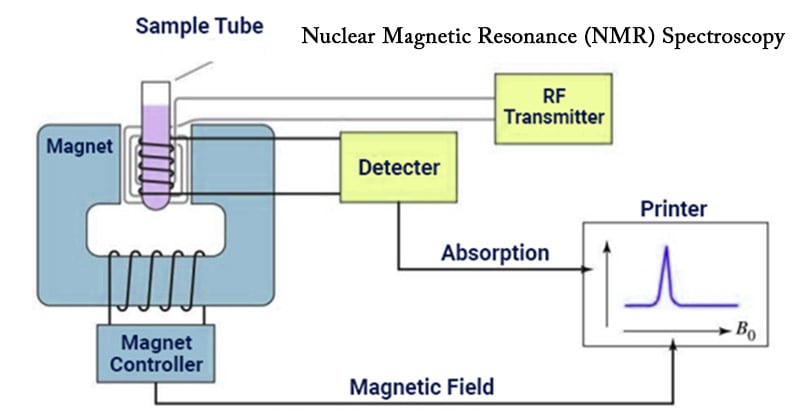
The basis of NMR spectroscopy lies in the fact that certain atomic nuclei possess a property called “spin,” which generates a magnetic moment. When placed in a strong external magnetic field, these nuclei can align with or against the field, resulting in two different energy states. By applying radiofrequency pulses to perturb the nuclear spins and then observing the energy absorbed or emitted during relaxation to their equilibrium states, valuable information about the surrounding environment can be obtained.
The NMR spectrum represents the absorption or emission of electromagnetic radiation in the radiofrequency region by the atomic nuclei within a sample. The spectral peaks in the NMR spectrum correspond to different atomic nuclei within the molecule, and their positions and intensities provide crucial information about the local chemical environment, connectivity, and molecular dynamics.
NMR spectroscopy is widely used in various scientific fields, including chemistry, biochemistry, and structural biology. It is particularly valuable for determining the structures of organic compounds, elucidating molecular conformations, and studying molecular interactions. Additionally, NMR can be used to analyze isotopic compositions and study the dynamics of biomolecules in solution.
One of the key advantages of NMR spectroscopy is its ability to provide a complete and detailed analysis of the entire spectrum. This allows researchers to obtain a wealth of information about a molecule’s structure and properties, making it an essential tool for structural determination and characterization in both academic and industrial research settings.
Nuclear Magnetic Resonance (NMR) spectroscopy has a wide range of practical applications in various scientific disciplines and industries. Some of the key applications include:
1. Structural Elucidation: NMR spectroscopy is extensively used to determine the structures of organic and inorganic compounds. It can provide detailed information about the connectivity of atoms in a molecule, bond lengths, and molecular conformations.
2. Drug Discovery and Development: NMR spectroscopy is crucial in drug development to study interactions between potential drug candidates and their target proteins. It helps researchers understand the binding interactions and optimize drug designs.
3. Protein Structure Determination: NMR spectroscopy is a powerful tool for studying the three-dimensional structures of proteins and peptides in solution. It is particularly useful for characterizing flexible or intrinsically disordered proteins.
4. Metabolomics: NMR spectroscopy is used in metabolomics studies to identify and quantify small molecules (metabolites) present in biological samples. It is employed in medical research, nutrition, and disease biomarker discovery.
5. Quality Control: NMR spectroscopy is used in industries like pharmaceuticals and food to assess the purity and quality of products. It can detect impurities and confirm the identity of compounds.
6. Environmental Analysis: NMR spectroscopy is applied to analyze environmental samples like soil, water, and air pollutants. It helps identify and quantify various organic and inorganic substances.
7. Petroleum and Petrochemical Industry: NMR is used to characterize petroleum products, including crude oil, gasoline, and polymers. It helps monitor product quality and assess the composition of complex mixtures.
8. Forensic Analysis: NMR spectroscopy can aid in forensic investigations by identifying and characterizing unknown substances found at crime scenes or in drug trafficking cases.
9. Material Science: NMR is used to study the structure and properties of materials such as polymers, catalysts, and nanomaterials. It provides insights into their composition and interactions.
10. Pharmaceutical Formulation: NMR spectroscopy is employed in pharmaceutical formulations to study drug-excipient interactions and ensure the stability of the final product.
11. Biological and Biochemical Research: NMR is used to investigate molecular interactions, study protein folding, and determine binding affinities between biomolecules.
12. Process Control: NMR can be integrated into industrial processes to monitor reactions, optimize reaction conditions, and control product quality in real-time.
Overall, NMR spectroscopy’s versatility and non-destructive nature make it an indispensable tool in numerous scientific and industrial applications, enabling researchers and industries to gain valuable insights into the structure and properties of diverse compounds and materials.
What are the limitations of NMR spectroscopy?
While NMR spectroscopy is a powerful and widely used analytical technique, it does have certain limitations and challenges. Some of the key limitations of NMR spectroscopy include:
1. Sensitivity: NMR spectroscopy requires a relatively large amount of sample compared to other spectroscopic techniques. The low sensitivity of NMR can be a limitation when dealing with small quantities or dilute samples.
2. Detection of Low-Abundance Species: In complex mixtures, low-abundance species may be challenging to detect and may get overshadowed by more abundant components, making their analysis difficult.
3. Resolution: In crowded spectra or samples with overlapping signals, spectral resolution can be reduced, leading to challenges in assigning and interpreting peaks accurately.
4. Spectral Complexity: For large and complex molecules, the NMR spectra can become intricate, and peak assignments can be time-consuming and complicated.
5. Instrument Cost and Maintenance: High-quality NMR instruments can be expensive to acquire and maintain, making access to advanced NMR facilities a limitation for some researchers.
6. Long Experiment Times: Some NMR experiments require significant acquisition times, especially for nuclei with low natural abundance, limiting the throughput and efficiency of data collection.
7. Limited Sensitivity for Some Nuclei: NMR spectroscopy is most commonly applied to nuclei with a magnetic moment, such as ^1H, ^13C, and ^31P. Other important nuclei, like ^15N, ^17O, and ^19F, have lower natural abundance and sensitivity, making their analysis more challenging.
8. Sample Solubility: NMR spectroscopy is typically performed in solution. Some compounds may be difficult to dissolve in suitable solvents or may require specialized NMR techniques like solid-state NMR.
9. Size Limitations: For very large biomolecules or macromolecular complexes, the size of the molecule can present challenges in acquiring interpretable NMR spectra.
10. Complexity of 2D and 3D Experiments: Advanced NMR techniques, such as 2D and 3D NMR experiments, can be time-consuming and require specialized expertise for data acquisition and analysis.
Despite these limitations, NMR spectroscopy remains a highly valuable technique for elucidating molecular structures, characterizing interactions, and gaining insights into molecular dynamics. Researchers often combine NMR with other spectroscopic and analytical methods to overcome these limitations and obtain a comprehensive understanding of complex systems. Additionally, ongoing advancements in NMR instrumentation and methodologies continue to address many of these challenges, enhancing the technique’s capabilities and applicability.