What is a peptide?
- A short chain made up of amino acids, which, together with other peptides, forms a protein.
- Peptide length can range from two to fifty amino acids.
- Classified based on length: oligopeptides (10 or fewer amino acids) and polypeptides (more than ten amino acids).
- Polypeptides with around 100 amino acids are considered proteins.
Peptide bond definition:
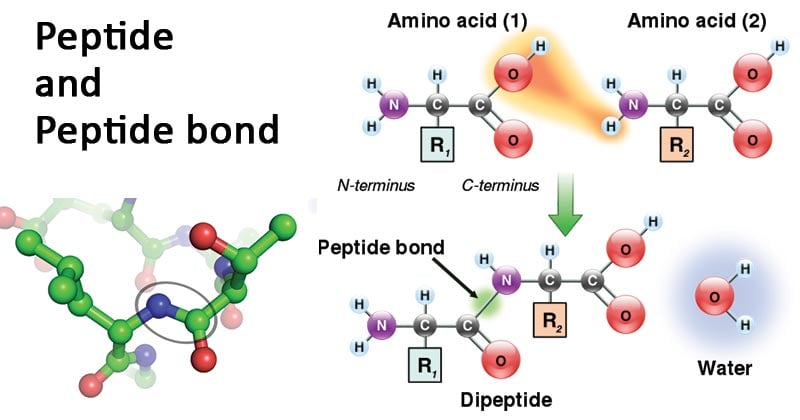
- A specific type of amide bond formed between two molecules.
- Occurs when the α-carboxyl group of one molecule reacts with the α-amino group of another molecule, releasing a water molecule.
- Also known as an isopeptide bond when formed between positions other than the alpha position.
- Formation involves a condensation reaction resulting in dehydration.
- Peptide bonds are covalent bonds that link amino acids in a peptide chain.
- Stabilized by a partial double bond between carbon and nitrogen in the amide bond.
Peptide bond formation mechanism:
- Involves a dehydration synthesis process.
- Carboxyl group of one amino acid moves towards the amino group of another amino acid.
- Results in the release of a water molecule and formation of an amide bond (C-N) between the two amino acids.
- Requires energy, often obtained from ATP in living beings.
Peptide bond degradation mechanism:
- Occurs through hydrolysis, requiring water molecules.
- Amide bond’s partial double bond slows down the degradation reaction.
- Water’s OH– ions attack the carbon atom, breaking the peptide bond.
- Hydrogen ion of water then attacks the nitrogen atom, cleaving the peptide into two units.
- Usually catalyzed by proteolytic enzymes like proteases and peptidases.
Peptide bond hydrolysis:
- Important step in protein-related processes.
- Involves breaking peptide bonds using water molecules, often catalyzed by acidic conditions.
- Essential for protein degradation, synthesis, and digestion in living organisms.
Examples:
- Peptide bonds present in all proteins, holding amino acids together in a chain.
- Peptides of different lengths: monopeptide (one amino acid), dipeptide (two amino acids), tripeptide (three amino acids), etc.
References:
- Jain JL, Jain S, and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition.
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company.
- ARLINGHAUS R, SHAEFER J, SCHWEET R. MECHANISM OF PEPTIDE BOND FORMATION IN POLYPEPTIDE SYNTHESIS. Proc Natl Acad Sci U S A. 1964; 51(6):1291-1299. DOI:10.1073/pnas.51.6.1291